
The removal of an electron from the 3 d sub-shell by photo-ionization leads to a (3 d) 9 configuration for the final state - since the d-orbitals ( l = 2) have non-zero orbital angular momentum, there will be coupling between the unpaired spin and orbital angular momenta. The inner core electronic configuration of the initial state of the Pd is : This arises from spin-orbit coupling effects in the final state. The 3 d photoemission is in fact split between two peaks, one at 334.9 eV BE and the other at 340.2 eV BE, with an intensity ratio of 3:2. We can see this more clearly if, for example, we expand the spectrum in the region of the 3 d emission.
the peak intensities are not simply related to the electron occupancy of the orbitalsĬloser inspection of the spectrum shows that emission from some levels (most obviously 3 p and 3 d ) does not give rise to a single photoemission peak, but a closely spaced doublet. there are significant differences in the natural widths of the various photoemission peaks. 330 eV (in this case it is really meaningless to refer to an associated binding energy). the remaining peak is not an XPS peak at all ! - it is an Auger peak arising from x-ray induced Auger emission. 335 eV is due to emission from the 3 d levels of the Pd atoms, whilst the 3 p and 3 s levels give rise to the peaks at ca. the emission from the 4 p and 4 s levels gives rise to very weak peaks at 54 eV and 88 eV respectively. 4 - 12 eV if measured with respect to the vacuum level ). 0 - 8 eV ( measured with respect to the Fermi level, or alternatively at ca. the valence band (4 d, 5 s) emission occurs at a binding energy of ca. Working downwards from the highest energy levels. The most intense peak is now seen to occur at a binding energy of ca. Since the photon energy of the radiation is always known it is a trivial matter to transform the spectrum so that it is plotted against BE as opposed to KE. the main peaks occur at kinetic energies of ca. The diagram below shows a real XPS spectrum obtained from a Pd metal sample using Mg K α radiation \[A + hν \rightarrow A^+ + e^- \label\): The XPS spectrum of Pd metal The process of photoionization can be considered in several ways : one way is to look at the overall process as follows : the number of emitted photoelectrons as a function of their kinetic energy) can be measured using any appropriate electron energy analyzer and a photoelectron spectrum can thus be recorded. The kinetic energy distribution of the emitted photoelectrons (i.e. By contrast, in UPS the photon interacts with valence levels of the molecule or solid, leading to ionization by removal of one of these valence electrons. In XPS the photon is absorbed by an atom in a molecule or solid, leading to ionization and the emission of a core (inner shell) electron. Photoelectron spectroscopy uses monochromatic sources of radiation (i.e. \(\nu\) is th e frequency (Hz) of the radiation. 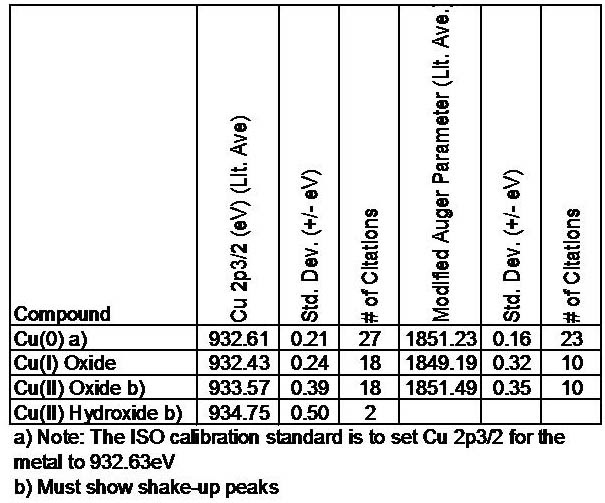
The energy of a photon of all types of electromagnetic radiation is given by the Einstein relation :
Photoelectron spectroscopy is based upon a single photon in/electron out process and from many viewpoints this underlying process is a much simpler phenomenon than the Auger process.






 0 kommentar(er)
0 kommentar(er)
